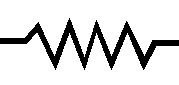2. Quartz Crystal: A slice of quartz grounded to a prescribed thickness that vibrates at a steady frequency when stimulated by electricity. The tiny crystal about 1/20th by 1/5th of an inch, creates the computer's heartbeat. It is piezoelectric, generate electricity when subjected to pressure.
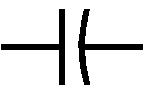 3. Resistors are used to lower the level of current in a closed loop.
3. Resistors are used to lower the level of current in a closed loop.4. Capacitors are electronic components used for storing small amount of electricity. That explains how radios keep playing for a couple of seconds even after unplugging them.
5. Amplifiers are used to make weak signals stronger.Amplitude modulated signal stays on one exact frequency.
6. Frequency modulated signal is transmitted by changing its frequency.FM radio waves always stay on the same amplitude.
7. The electricity running through human's brain is of too small a magnitude to be measured by most electrical equipments.
8. To generate a radio wave, electric field must be continuously changing.
9. Electronics is a branch of physics that deals with how electrons behave in vacuum or gases.Electricity is the group name of energy possessed by energized electrons.
10. Electronic systems normally use relatively small amounts of electric energy to process or transmit information.
11. Homes usually have AC supply.Electronic components have a transformer to convert AC to DC.